EH&S is currently fulfilling the packaging and shipping needs for the UC Merced student COVID-19 testing program and the asymptomatic on-site employee testing program. All staff involved in packaging and shipping have been trained in IATA Biological Dangerous Goods Shipping, per IATA/DOT requirements.
If you have any needs related to SARS-CoV-2 packaging, shipping, or transportation, please contact the EH&S biosafety group.
The OSHA Bloodborne Pathogens Standard (29 CFR 1910.1030) applies when handling/shipping human sourced materials.
United States
Per CDC and DOT regulations, suspected and confirmed SARS-CoV-2 patient specimens, cultures, or isolates must be packaged and shipped as UN 3373 Biological Substance, Category B, in accordance with the current edition of the International Air Transport Association (IATA) Dangerous Goods Regulations and U.S. Department of Transportation’s (DOT) Transporting Infectious Substances Safely.
International
According to the World Health Organization's Laboratory Biosafety Guidance Related to Coronavirus Disease (COVID-19), patient specimens from suspected or confirmed cases should be transported as UN3373, “Biological Substance Category B”. Viral cultures or isolates should be transported as Category A UN2814, “Infectious Substance, Affecting Humans”.
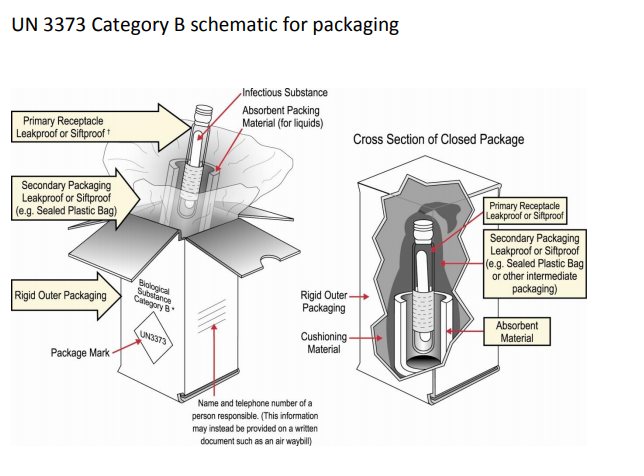
Note: Any courier can refuse to transport a package that they feel does not meet their packaging standards for shipment of biological substances or patient samples; therefore, it is advisable to review packaging and labeling with the vendor first.
Additional Resources
IATA Novel Coronavirus (COVID-19) Guidance for Operators
COVID-19 Testing Information



